- Anoop, B. S., Puthumana, J., Vazhappilly, C. G., Kombiyil, S., Philip, R., Abdulaziz, A., & Singh, I. S. B. (2021). Immortalization of shrimp lymphoid cells by hybridizing with the continuous cell line Sf9 leading to the development of ‘PmLyO-Sf9’. Fish & Shellfish Immunology, 113, 196-207.
- Balakrishnan, S., Singh, I. S., & Puthumana, J. (2022). Status in molluscan cell line development in last one decade (2010–2020): impediments and way forward. Cytotechnology, 1-25.
- Bhaskaran Sathyabhama, A., Puthumana, J., Sukumaran, V., Vazhappilly, C. G., Kombiyil, S., Philip, R., & Singh, I. S. B. (2021). A Novel Approach of Transducing Recombinant Baculovirus into Primary Lymphoid Cells of Penaeus monodon for Developing Continuous Cell Line. Marine Biotechnology, 23(4), 517-528.
- Jayesh, P., Jose, S., Philip, R., & Bright Singh, I. S. (2013). A novel medium for the development of in vitro cell culture system from Penaeus monodon. Cytotechnology, 65(3), 307-322.
- Jayesh, P., Philip, R., & Singh, I. S. (2015). Multifactorial interaction of growth factors on Penaeus monodon lymphoid cells and the impact of IGFs in DNA synthesis and metabolic activity in vitro. Cytotechnology, 67(3), 559-571.
- Jayesh, P., Seena, J., & Singh, I. S. (2012). Establishment of shrimp cell lines: perception and orientation. Indian Journal of Virology, 23(2), 244-251.
- Jayesh, P., Vrinda, S., Priyaja, P., Philip, R., & Singh, I. S. (2016). Impaired telomerase activity hinders proliferation and in vitro transformation of Penaeus monodon lymphoid cells. Cytotechnology, 68(4), 1301-1314.
- Jose, S., Jayesh, P., Mohandas, A., Philip, R., & Singh, I. B. (2011). Application of primary haemocyte culture of Penaeus monodon in the assessment of cytotoxicity and genotoxicity of heavy metals and pesticides. Marine Environmental Research, 71(3), 169-177.
- Jose, S., Jayesh, P., Sudheer, N.S., Poulose, G., Mohandas, A., Philip, R., Bright Singh, I.S., 2012. Lymphoid organ cell culture system from Penaeus monodon (Fabricius) as a platform for white spot syndrome virus and shrimp immune-related gene expression. J. Fish Dis. 35, 321–334. https://doi.org/10.1111/j.1365-2761.2012.01348.x
- Jose, S., Mohandas, A., Philip, R., & Singh, I. B. (2010). Primary hemocyte culture of Penaeus monodon as an in vitro model for white spot syndrome virus titration, viral and immune related gene expression and cytotoxicity assays. Journal of invertebrate pathology, 105(3), 312-321.
- Puthumana, J., Jose, S., Philip, R., & Singh, I. B. (2015). Cellular and molecular markers in monitoring the fate of lymphoid cell culture from Penaeus monodon Fabricius (1798). Fish & Shellfish Immunology, 47(2), 893-901.
- Puthumana, J., Philip, R., & Bright Singh, I. S. (2016). Transgene expression in Penaeus monodon cells: evaluation of recombinant baculoviral vectors with shrimp specific hybrid promoters. Cytotechnology, 68(4), 1147-1159.
- Puthumana, J., Prabhakaran, P., Philip, R., & Singh, I. B. (2015). Attempts on producing lymphoid cell line from Penaeus monodon by induction with SV40-T and 12S EIA oncogenes. Fish & Shellfish Immunology, 47(2), 655-663.
- Sathyabhama, A. B., Puthumana, J., Kombiyil, S., Philip, R., & Singh, I. S. B. (2021). ‘PmLyO-Sf9-WSSV complex’could be a platform for elucidating the mechanism of viral entry, cellular apoptosis and replication impediments. Virology, 553, 102-110.
Cell culture and cell lines have assumed an important role in studying physiological, pathophysiological, and differentiation processes of specific cells. It allows the examination of stepwise alterations in the structure, biology, and genetic makeup of the cell under controlled environments.
With the deepening of economic globalization, people’s demand for aquatic products is gradually increasing. Aquaculture practitioners began to pursue high yield and high income, but the emergence of this large-scale and high-density farming technology will inevitably accelerate the transmission and spread of diseases in water. In order to better this problem, cellular models are needed for studying fish growth, disease, reproduction, genetics, and biotechnology.
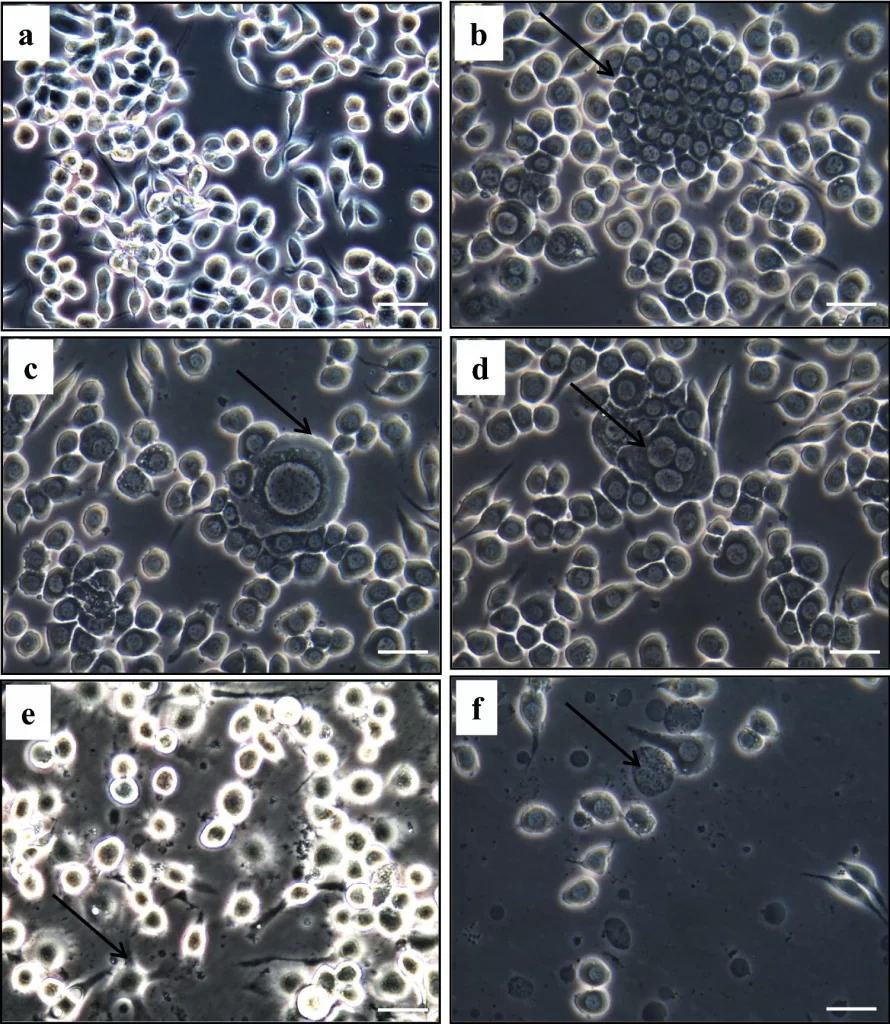
A cell line, in simple terms, refers to a defined population of cells capable of proliferating indefinitely in vitro. The path toward developing a cell line starts with the development of primary cell culture, which is derived from a specific tissue of the donor animal. Many factors come into consideration while choosing the donor animal such as age, health condition, etc. The type of tissue chosen depends on the application intended for the developed cell line. For example, hemocyte culture can be used for applications in immunology. Other critical factors are the inherent ability of the cells to proliferate (hemocytes and heart cells have limited capacity for proliferation whereas stem cells and germ cells have good proliferation capacity), and lesser chances of contamination by bacteria, fungi, etc. in the primary cell culture (gills being in continuous contact with the external environment have more chances to harbour bacteria and fungi, whereas internal organs tend to more aseptic in nature).
The cell culture medium in which cells are cultured should be able to provide all the necessary factors required by the cells to survive, proliferate, and carry out metabolic activities. Therefore, the development/optimization of organism/tissue-specific cell culture medium is itself a research area of importance. The cell culture conditions provided in vitro such as pH, temperature, etc. should be as close as possible to the physiological conditions.
Certain cell culture systems undergo spontaneous immortalization in vitro leading to the development of a continuous/immortal cell line. Carcinogenic primary cell cultures also often develop into continuous cell lines. Immortalization can also be induced in cell culture systems by way of transfecting the cells with viral oncogenes or by developing hybrid cells (fusion of primary cells with those from a continuous cell line).
Aquatic animal health and disease management are now studied from a variety of angles, such as environmental protection and pollution control, human health and epidemiology, site selection and culture technologies, monitoring and sanitation of aquaculture facilities, diagnosis and treatment of diseases of cultured species, avoidance of nutritional diseases, prevention of epidemics of mortality in culture facilities, formulation and implementation of regulatory measures, and more. Aquatic farm animals, in contrast to terrestrial farm animals, require greater care to monitor their health because they live in a complex and dynamic habitat and are not easily visible except under tank holding settings.
Aquatic animal health management is one of the core research themes of the centre. The research team focusing on bacterial, viral, fungal and protozoan and metazoan parasitic diseases of finfish and shellfishes and it’s epidemiology, pathology, host-pathogen interaction, immunology, development of management measures for the control of diseases such as vaccine development, probiotics, bioremediation, prophylactic measures etc. the team working on the development of novel diagnostic tools for the detection and identification of pathogens.
Recent Updates
- Immortalization of shrimp lymphoid cells by hybridizing with the continuous cell line Sf9 leading to the development of ‘PmLyO-Sf9 ’
- A Novel Approach of Transducing Recombinant Baculovirus into Primary Lymphoid Cells of Penaeus monodon for Developing Continuous Cell Line
- A novel medium for the development of in vitro cell culture system from Penaeus monodon
Research Projects
- Cell and developmental biology of Marine organisms funded by Department of Biotechnology, Govt of India
- Immortalization of lymphoid cell culture from Penaeus monodon by transduction with oncogenes, ectopic expression of telomerase reverse transcriptase using shrimp specific expression vectors and hybridization with established cell lines funded by Department of Biotechnology, Govt of India.
- Identification and removal of blocks on in vitro transformation and establishment of cell lines from Penaeus monodon funded by Department of Biotechnology, Govt of India.
- Development of cell culture systems from penaeid prawns for the isolation of White Spot Virus funded by Department of Biotechnology, Govt of India.
- Development of primary cell cultures and cell lines from fresh water fishes to isolate viruses associated with EUS funded by State Committee on Science, Technology & Environment, Govt. of Kerala
Research Publications
- Anoop B.S. Establishment of Shrimp- Insect Hybrid Cell Line PmLyO –Sf 9 and Development of Transduction Based Oncogenic Induction for Shrimp Cell Immortalisation. view
- Jayesh P. 2013. Development of lymphoid cell culture system from Penaeus monodon and molecular approaches for its transformation. view view
- Seena Jose. 2009. Cell Culture Systems from Penaeus monodon: Development and Application. view
- Sunil Kumar G. 2000. Development of Cell Culture Systems from Selected Species of Fish and Prawns. view view
Thrust Areas
- Development of cell culture systems from freshwater and marine fish.
- Development of cell culture systems from freshwater and marine invertebrates.
- Use of CRISPR /cas9 technology for genome editing.
- Transgenic experiments in cell culture systems.